Abstract
Interleukin 1 beta (IL-1B) induced inflammasome activation is a mechanism of cancer initiation and progression (Schroder K, Cell, 2010). Our previous studies (Ganan-Gomez I, Blood 2021) showed that IL-1B was one of the most overexpressed pro-inflammatory cytokines in the hematopoietic stem and progenitor cells (HSPCs) of patients with clonal cytopenia of undetermined significance (CCUS) carrying DNMT3a and TET2 mutations. Given that IL-1B upregulation induces aberrant myeloid differentiation of HSPCs (Pietras EM, Nat Cell Biol., 2016), we hypothesize that IL-1B plays a critical role in MDS pathogenesis, and that targeting IL-1B signaling overcomes aberrant MDS HSPCs' myeloid skewing. To test this hypothesis, we designed a clinical trial to evaluate the IL-1B inhibitor canakinumab's (NCT04239157) safety profile and its clinical and biological effects in patients with lower-risk MDS.
Patients and Methods: Patients aged ≥ 18 with IPSS-R ≤ 3.5 MDS, normal renal and hepatic functions, and performance status, who had previously received at least 1 line of other therapy were included in the study (Table1). Patients received 300 mg of canakinumab on day 1 of a 4-week cycle. Bone marrow (BM) samples were obtained before therapy and after 1, 2, and 4 cycles of therapy during the trial and after every 3 or 4 cycles of therapy thereafter. Single-cell RNA sequencing (scRNA-seq; 10X Genomics) analyses were performed in lineage negative (Lin-)CD34+ HSPCs and mononuclear cells (MNCs) isolated at different therapy timepoints from the BM of 1 patient who achieved hematological improvement after canakinumab treatment, 2 patients who achieved stable disease, and 2 patients who did not respond to this therapy. The clinical characteristics of the 5 patients whose samples were analyzed by scRNA-seq analyses are included in Table 1.
Results: Twenty-six patients have been enrolled in the clinical trial. Patients' clinical characteristics are included in Table 1. Twenty-three patients were evaluable for response. Among the 22 out of the 23 patients who were transfusion dependent at baseline, one achieved transfusion independence, and another had platelet count improvement. Three out of the 23 had progressive disease, and 18 had stable disease. Treatment was well tolerated with no severe adverse events. One patient died of sepsis unrelated to therapy. Overall, we did not observe any improvement in the blast counts and cytogenetic and/or mutational burden.
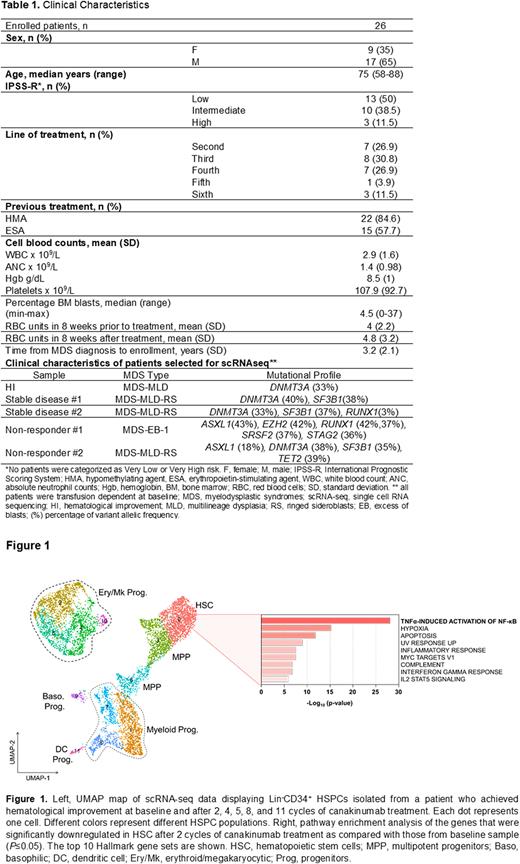
To evaluate the biological effects of canakinumab, we performed scRNA-seq of Lin-CD34+ HSPCs (Figure 1) and MNCs at therapy baseline and after 2, 4, 5, 8, and 11 cycles of treatment. Consistent with our initial hypotheses, these analyses revealed that canakinumab decreased the frequencies of hematopoietic stem cells (HSCs) and multipotent myeloid progenitors (MPPs). Differential analysis between the samples showed that HSCs and MPPs significantly downregulated the expression of genes involved in cytokine signaling (e.g., IL-1B, CXCL2, and CXCL8), TNFα-induced activation of NF-κB, and hypoxia pathways, which demonstrated efficient target engagement of canakinumab on MDS cells. Similar results were also observed in downstream monocytes and B cells. In contrast, we did not observe any significant change in the HSPC architecture in the BM of patients with stable disease or whose disease did not respond to canakinumab. Consistent with our previous results in early stage MDS, the patient who achieved hematological improvement after canakinumab therapy was the only one among the cohort who carried a single mutation affecting DNMT3A (Table1).
Conclusion. This study demonstrates that canakinumab is safe in MDS and efficiently targets IL-1B signaling. However, the biological effect of canakinumab has not yet translated in clinical responses in the subset of patients enrolled in this clinical trial. The study will accrue transfusion independent patients and patients with early stage MDS as CCUS.
Disclosures
Garcia-Manero:Gilead Sciences: Research Funding; Genentech: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; AbbVie: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; Acceleron Pharma: Consultancy; Curis: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees. Takahashi:Symbio Pharmaceuticals: Consultancy; Ostuka Pharmaceuticals: Honoraria; Agios: Consultancy; Celgene/BMS: Consultancy; GSK: Consultancy; Novartis: Consultancy; Mission Bio: Honoraria; Illumina: Honoraria. Short:Amgen: Consultancy, Honoraria; Stemline Therapeutics: Research Funding; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Novartis: Consultancy; AstraZeneca: Consultancy; Pfizer: Consultancy. Issa:Novartis, Kura Oncology, Nuprobe: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding. Colla:Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.